- You do not have any products in your shopping cart yet.
Mutations
Green Spring Statement on the detection of mutant viruses
Date: August 30, 2024< br />
Product: GREEN SPRING® SARS-CoV-2 antigen rapid test kit (colloidal gold)
Product: GREEN SPRING Corona Test, 40 Boxes x 25, 4in1 - Professional, 1 box = 1,000 tests
We, Shenzhen Lvshiyuan Biotechnology Co. Ltd. as manufacturer, hereby declare that the Green Springs SARS-CoV -2 Antigen Rapid Test Kit (Colloidal Gold) can detect the following SARS-CoV-2 virus variants, which are classified by the World Health Organization as "variants of concern (VOC)", "variants of interest (VII), VOI)" and "variants under monitoring (VUM)" VOC refers to the large number and wide range of cases caused worldwide, and the data confirm the transmissibility, strong toxicity or reduced effectiveness of vaccines and clinical treatments. VOI refers to a confirmed case of community transmission or has been found in multiple countries but has not yet formed a widespread infection. VUM refers to a SARS-CoV-2 variant with genetic changes suspected of affecting the virus's properties, suggesting it may pose a risk in the future. However, the phenotypic or epidemiological impact is currently unclear, so increased surveillance and reassessment are required until new evidence becomes available.
| WHO categories | WHO label | Pango lineage | Date of designation |
|---|---|---|---|
| Variants of concern (VOC) | Alpha | B.1.1.7 | Sep 20 |
| Beta | B.1.351 | May 20 | |
| Gamma | P.1 | Nov 20 | |
| Delta | B.1.617.2 | Oct 20 | |
| Omicron | B.1.1.529 | Nov 21 | |
| Variants of interest ( VOI) | Epsilon | B.1.427 | Feb 21 |
| B.1.429 | Jun 21 | ||
| Eta | B.1.525 | Feb 21 | |
| Iota | B.1.526 | Feb 21 | |
| Kappa | B.1.617.1 | May. 21 | |
| N/A | B.1.617.3 | May 21 | |
| Zeta | P.2 | Feb 21 | |
| Mu | B.1.621, B.1.621.1 | Sep 21 | |
| IHU | B.1.640.2 | Sep 21 | |
| & Uuml; monitored variants (VUMs) | BA.1 xAY.4 recombinant | Mar 22 | |
| B.1.640 | Nov 21 | ||
| BA.1 | Nov 21 | ||
| BA.2 | Feb 22 | ||
| BA.2.12.1 | Feb 22 | ||
| BA.3 | Feb 22 | ||
| BA.4 | Jan 22 | ||
| BA.5 | Jan 22 | ||
| BF.7 | Mar 22 | ||
| BA.2.75 | May 22 | ||
| BQ.1.1 | Aug 22 | ||
| XBB | Sep 22 | ||
| BQ.1 | Sep 22 | ||
| EG.5 | Feb 23 | ||
| Pirola(BA.2.86) | Aug 23 | ||
| JN.1 | Aug 23 | ||
| KP.2 | Feb 24 |
The new coronavirus (SARS-CoV-2 or 2019-nCoV) is a non-segmented forward RNA virus.
It is the cause of the new type of coronavirus pneumonia (COVID-19), which is highly contagious to humans. The SARS-CoV-2 virus has several structural proteins, including spikes (S), envelope (E), membrane (M) and nucleocapsid (N).
The SARS-CoV-2 virus is characterized by a high stability of the nucleocapsid protein (N).
The mutated virus strains found worldwide are derived from the SARS-CoV-2 20B/GR (lineage B.1.1.7) and contain many mutations, with the mutation in the spike protein (S protein) of the new coronavirus, which is the site where the SARS-CoV-2 virus binds to the cell's ACE2 receptor.
The SARS-CoV-2 Antigen Rapid Test Kit manufactured by Shenzhen Lvshiyuan Biotechnology Co., Ltd. is intended for the qualitative in vitro detection of the nucleocapsid (N) protein of the SARS-CoV-2 virus in human nasopharyngeal, oropharyngeal, anterior nasal or saliva samples.
It is shown that the mutation sites of the mutated virus strains, including the EG.5 strain, have no influence on the detection rate of the kits we produce. The kit is suitable for the detection of the SARS-CoV-2 variant as listed in the table above.
Fluorecare Statement on the detection of mutant viruses
Date : December 22nd, 2022
Product: FLUORECARE® RSV test
Product: FLUORECARE Corona, Influenza & RSV test, 1 layperson, 1 box = 500 tests
To all who may be concerned
We, Shenzhen Microprofit Biotech Co., Ltd. as the manufacturer of the SARS-CoV-2 Mutation Impact on SARS-CoV-2 & Influenza A/B & RSV Antigen Combo Test Kits (Colloidal Gold Chromatographic Immunoassay)(Self-test)(SOLMIRA - REF MF-71) hereby declare,
1. Further in vitro studies to investigate the effects of mutated SARS-CoV -2 N-proteins and the analytical performance of the product are completed, B.1.1.7, B.1.351, B.1.429, B.1.427, B.1.2, P1, P2, B.1.617.2, B.1.617.3 , C.37, P.3, B.1.617. 1, B.1.525, B.1.526.1, B.1.526.2, B.1.1.529, BA.1, BA.2, XE, BA.4, BA.5, BA.4.6, BF.7, BA.2.75, B.1.1.318,C.36.3,AY.4.2,BA.2.10,XBB,XBC , BQ.1,BQ.1.1,BJ.1,BA.2.3.20,B.1.621, BM. 2, BA.4.7, BN.1, BA.5.2.35, BQ.1.4, BQ.1.3, BQ.1.2, BA.5.9, BM.1.1.1, BS.1, BA.2.75.5, BA.2.75.2, BA.2.12.1, variants where the N protein mutation site is not in the recognition region of the coated and labeled antibody can be easily recognized by the reaction.
2. In the future our company will continue to track the mutation of the new coronavirus and timely evaluate and verify the detection ability of the mutated recombinant protein and clinical performance to ensure the sensitivity and specificity of the detection kit.
Hotgen Statement for the detection of mutant viruses
Date : 08/29/2023
Product: HOTGEN SLEEVE Corona Test, 1 nasal layperson
For the product Coronavirus (2019-nCoV) antigen test from Beijing Hotgen Biotech Co., Ltd., the following statements apply in particular:
Our company's Coronavirus (2019-nCoV) antigen test detects the N protein of SARSCoV-2 in human anterior nasal swab samples. While the mutation of the virus that emerged in
England (B.1.1. 7 strain),South Africa (B.1.351 strain, Omicron variant (B.1.1.529 strain, BA.2 strain, BA .2.75 strain, BA.4 strain, BA.5 strain, BF.7 strain, BF.15 strain, BQ.1 strain, BQ.1.1 strain, XBB strain & XBB.1 strain)),Brazil (P.1 strain), India (B.1.617 strain, Delta variant B.1.617.2)XE variant, EG.5 variant and BA.2.86 are mainly based on S protein.So our product is still capable of detecting the antigen of novel coronaviruses, and the mutation has no influence on the detection result of our product.
Hightop Statement on the detection of mutant viruses
Date : June 14th, 2022
Product: HIGHTOP Corona Test, 1 nasal layman
The SARS-COV-2 antigen rapid test from Qingdao Hightop Biotech Co. Ltd. detects the nucleocapsid protein (N protein). It does not target the spike protein (S protein) of the genetic SARS-CoV-2 variants (found in England (B.1 .1.7 strain), South Africa (B .1.351 strain, Omicron variant B.1.1529 strain), Brazil (P.1 strain) and India (B.1.617 strain, Delta variant B.1.617.2 strain). Therefore, mutations in the spike protein theoretically have no influence on the performance of the product, and our test can detect the mutated SARS-CoV-2 strains.
We will actively follow information about mutated SARS-CoY-2 strains and will make an evaluation in the near future of our test kits in the near future.
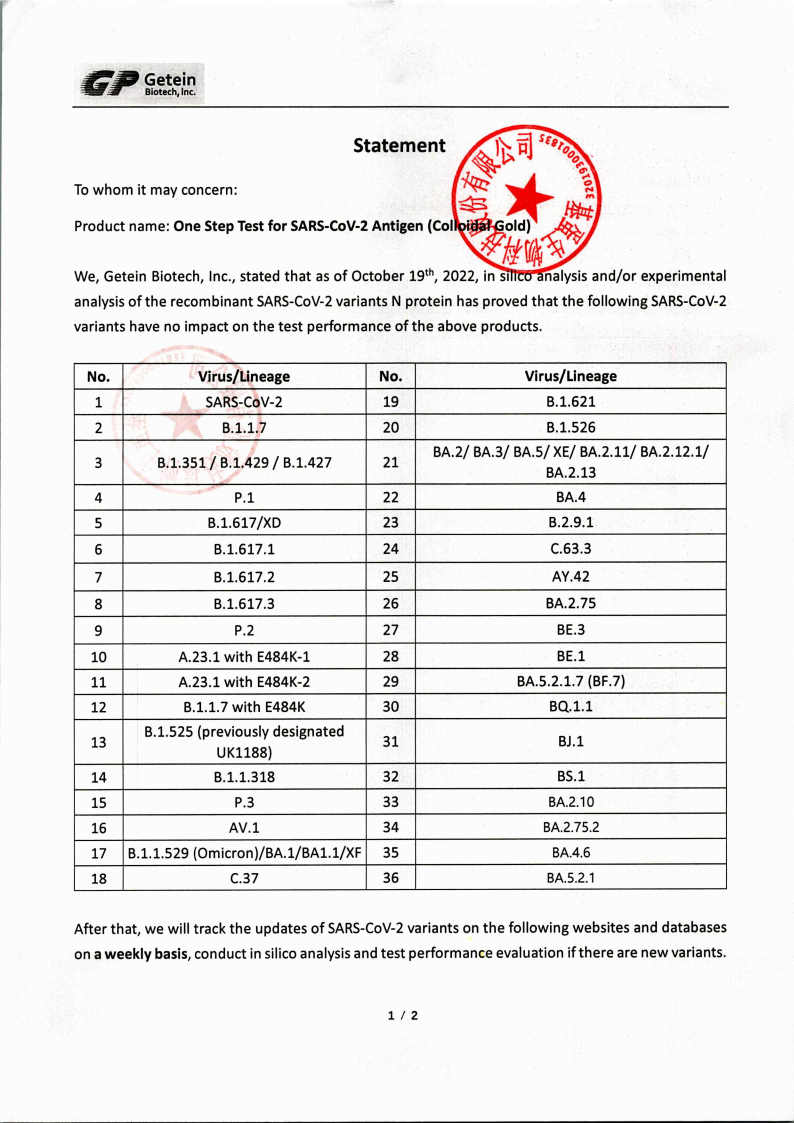
Getein Statement on the detection of mutant viruses
Date : October 19, 2022
Product: GETEIN Corona Test, 1 nasal layman
Product: GETEIN Corona Test, 5x Nasal Laie
Product: GETEIN Corona Test, 25x 1er Nasal Laie
Getein Biotech, Inc. declares that as of October 19, 202 rs and/or experimental analyses of the recombinant SARS-CoV-2 variant N protein have demonstrated that the The following SARS-CoV-2 variants have no influence on the test performance of the above-mentioned products.
Chil Statement on the detection of mutant viruses
Date : 06.01.2023
Product: CHIL Comfortcheck, 1 nasal - layperson
The spike protein of the Omicron variant is characterized by at least 30 amino acid substitutions, three small Deletions and a small insertion. In particular, 15 of the 30 amino acid changes are in the receptor binding domain (RBD). There are also a number of changes and deletions in other genomic regions.
Longsee Statement on the detection of mutant viruses
Date : 08.06.2022
Product: LONGSEE Corona Test, 25 Nasal Lay
To all who are concerned,
in response to the recent update of several variants of the novel coronavirus We, Guangdong Longsee Biomedical Co.,Ltd. as manufacturer of the 2019-nCo V Ag Rapid Detection Kit (Immuno-Chromatography) (LS-C-T-008/009), hereby declare that these products are suitable for the detection of SARS-Co V-2 antigens remain effective in the variants discovered. The variants include, among others, the new coronavirus variants such as
- Alpha (B.1.1.7),
- Beta (B.l.351/B.1.351.2/B.l.351.3) ,
- Gamma (P.l/P.l.l/P.1.2),
- Delta (B.1 .617.2/ A Y.1/A Y.2),
- < li>Eta(B.1.525), lota(B.1.526),
- Kappa(B.1.617.1),
- Lambda(C.37),
- Zeta(P.2),
- Theta(P.3),
- Epsilon (B.1.427/B.1.429),
- and Omicron(B.1.1.529/BA.2.12.1/BA.4/BA.5)
- etc.< /li>
We thank you for choosing LONGSEE products. We will continue to track and update the latest viral information, including mutations and substitutions for SARS-Co V-2, to improve the specificity and sensitivity of our products and strengthen our research and development, to provide you with high quality products and services.
Rightsign Statement on the detection of mutant viruses
Date: August 17, 2023
Product: RIGHTSIGN Corona Test, 1 Nasal Layman
To all those concerned:
This letter declares the detection of new COVID-19 mutant strains using the COVID-19 antigen rapid test cassette from Hangzhou Biotest Biotech Co. Ltd.
The test cassette manufactured by Hangzhou Biotest Biotech Co., Ltd. manufactured COVID-19 Antigen Rapid Test Cassette is a qualitative lateral flow immunoassay for the detection of the nucleocapsid protein of SARS-CoV-2.
Comparing the mutations found on the nucleocapsid protein of the variants (BA.1, BA.2, BA2.10, BA.2.12, BA.2.12.1, BA.2.75, XE, BF.7, BJ.1, XBB, BA.2.75.2, BN.1, BA.5.2.35, XBB.1.5, CH.1.1, CH1.1.1, CH1.1.2, XBF(BA.5 x CJ.1), XBB.1.9.1, XBB.1.9.2, XBB.1.16, XBB.2, XBB.2.3, XBB.2.3.2, XBB.1.16.1, XBB.l, FL.3 (XBB.1.9.1.3), XBB.1.5.7, FL.3.1, FL.4, FL.3.3, FY.1.2 and EG.5.1.) and the epitopes (the part of the antigen that binds to the antibody) of the antibodies, it was found that all mutations are outside the epitopes of the antibodies used in the test. This means that the binding of the antibodies to the antigen is unlikely to be affected, so the risk of the new variant being missed by the COVID-19 Antigen Rapid Test Cassette is low.
Comparing the mutations found on the nucleocapsid protein of the new variant B.1.617.2(Delta), BA.4, BA.4.6, BA.5, BE.3, BQ.1, BQ.1.1, BQ.1 .1.20 and the epitopes (the part of the antigen that binds to the antibody) of the antibodies used for the test for the variants B.1.617.2(Delta), BA.4, BA.4.6, BA.5, BQ.1,BQ.1.1 and BQ.1 .1.20, there was a change in the amino acid (AA) in the overlap of the epitope(s) sites of the anti-SARS-CoV-2 antibody used for used to coat the membrane and no change in the overlap of the anti-SARS-CoV-2 antibody used for conjugation, in variant BE.3, there were two amino acid changes in the overlap of the epitope positions of the anti-SARS-CoV-2 antibody used for coating the membrane and no variation in the overlap of the anti-SARS-CoV-2 antibody used for conjugation. From this it can be concluded that the COVID-19 antigen rapid test cassette is largely able to detect the antigen.
The inactivated virus B.1.617.2(Delta) was tested under laboratory conditions. laboratory conditions, and the results showed that the intensity of the T-line at different protein concentrations was consistent with that of the wild strain when tested with the COVID-19 Antigen Rapid Test Cassette.
The recombinant N-protein antigen of the new variant (BA.2, BA.2.10, BA.2.12, BA.2.12.1, XE, XBB, BA.2.75, BA.2.75.2, BN.l(BA.2.75.5.1), BA.5.2.35, XBB.1.5, CH.1.1, CHl.1.1, CHl.1.2 ,XBF(BA.5 x CJ.1), XBB.1.9.1, XBB.1.9.2, XBB.2, EG.5.1, BA.4, BA.4.6, BA.5, XBB.2.3, XBB.2.3.2, XBB.1.16.1, XBB.1, FL.3 (XBB.1.9.1.3), XBB.1.5.7, FL.3.1, FL.4, Fl.3.3 ,FY.1.2, BE.3, BQ.l, BQ.1.1 and XBB.1.16) was tested. The results showed that the intensity of the T line at different protein concentrations was consistent with that of the wild strain when tested with the COVID-19 Antigen Rapid Test Cassette.
From the above, it is clear that the COVID-19 Antigen Rapid Test Cassette is effective against virus mutations (B.l.617.2(Delta), BA.1, BA.2, BA2.10, BA.2.12, BA.2.12.1, BA.4, BA.4.6, BA.S, BA.2.75, XE, BE.3, BF.7, BQ.l, BQ.1.1, BJ.l, ,CHl.l.l, CHl.1.2, XBF(BA.5 x CJ.l), BQ.1.1.20, XBB.1.9.1, XBB.1.9.2, XBB.1.16, EG.l, 7, FL.3.1, FL.4, FL.3.3, FY.1.2, EG.5.l(XBB.1.9.), the risk of the new variant not being detected by the COVID-19 rapid antigen test cassette is low.
Our company will continue to closely monitor the situation of virus mutations at home and abroad, and timely evaluate the detection ability and product performance of our products on mutated strains to ensure that the accuracy and sensitivity of the COVID-19 rapid antigen test cassette are not affected. If necessary, the product will be quickly updated to ensure the accuracy of the COVID-19 rapid antigen test cassette on SARS-CoV-2 variants.
We thank you for your support and trust in our COVID-19 rapid antigen test cassette products.
Newgene Statement on the detection of mutant viruses
Date : October 19, 2022
Product: NEWGENE Corona Test 5, nasal - layman
To whom it may be addressed,
We, New Gene (Hangzhou) Bioengineering Co, Ltd. is the official developer and manufacturer of our proprietary COVID-19 Antigen Detection Kit and has factories located at Room 1606, Floor 16, Building 5, 688 Bin'an Road, Changhe Street, Binjiang District, Hangzhou (310051 ), Zhejiang, PR China, and hereby confirms that the COVID-19 Antigen Detection Kit detects the nucleocapsid antigens of SARS-CoV-2 and Omicron variants including BA.1, BA.2, BA.2.12 .1, BA.2.75, BA.3, BA.4, BA.5, BF.7, BQ.1.1, XBB. New Gene (Hangzhou) Bioengineering Co., Ltd. reserves all rights to the interpretation of this Writing.
Rightsign Statement on the detection of mutant viruses
Date : August 17, 2023
Product: RIGHTSIGN Corona Test, 1 Nasal Layman
To all those concerned:
This letter declares the detection of new COVID-19 mutant strains using the COVID-19 antigen rapid test cassette from Hangzhou Biotest Biotech Co. Ltd.
The rapid test cassette manufactured by Hangzhou Biotest Biotech Co., Ltd. manufactured COVID-19 Antigen Rapid Test Cassette is a qualitative lateral flow immunoassay for the detection of the nucleocapsid protein of SARS-CoV-2.
Comparing the mutations found on the nucleocapsid protein of the variants (BA.1, BA.2, BA2.10, BA.2.12, BA.2.12.1, BA.2.75, XE, BF.7, BJ.1, XBB, BA.2.75.2, BN.1, BA.5.2.35, XBB.1.5, CH.1.1, CH1.1.1, CH1.1.2, XBF(BA.5 x CJ.1), XBB.1.9.1, XBB.1.9.2, XBB.1.16, XBB.2, XBB.2.3, XBB.2.3.2, XBB.1.16.1, XBB.l, FL.3 (XBB.1.9.1.3), XBB.1.5.7, FL.3.1, FL.4, FL.3.3, FY.1.2 and EG.5.1.) and the epitopes (the part of the antigen that binds to the antibody) of the antibodies, it was found that all mutations lie outside the epitopes of the antibodies used in the test. This means that the binding of the antibodies and the antigen is unlikely to be affected, so the risk of the new variant not being detected by the COVID-19 Antigen Rapid Test Cassette is low.
Comparing the mutations found on the nucleocapsid protein of the new variant B.1.617.2(Delta), BA.4, BA.4.6, BA.5, BE.3, BQ.1, BQ.1.1, BQ.1 .1.20 and the epitopes (the part of the antigen that binds to the antibody) of the antibodies used for the test for the variants B.1.617.2(Delta), BA.4, BA.4.6, BA.5, BQ.1,BQ.1.1 and BQ.1 .1.20, there was one amino acid (AA) change in the overlap of the epitope(s) sites of the anti-SARS-CoV-2 antibody used for coating the membrane and no change in the overlap of the anti-SARS-CoV-2 antibody used for conjugation, in variant BE.3, there were two amino acid changes in the overlap of the epitope positions of the anti-SARS-CoV-2 antibody used for coating the membrane and no variation in the overlap of the anti-SARS-CoV-2 antibody used for Conjugation was used. This indicates that the COVID-19 antigen rapid test cassette is largely capable of detecting the antigen.
The inactivated virus B.1.617.2(Delta) was tested under laboratory conditions. laboratory conditions, and the results showed that the intensity of the T-line at different protein concentrations was consistent with that of the wild strain when tested with the COVID-19 Antigen Rapid Test Cassette.
The recombinant N-protein antigen of the new variant (BA.2, BA.2.10, BA.2.12, BA.2.12.1, XE, XBB, BA.2.75, BA.2.75.2, BN.l(BA.2.75.5.1), BA.5.2.35, XBB.1.5, CH.1.1, CHl.1.1, CHl.1.2 ,XBF(BA.5 x CJ.1), XBB.1.9.1, XBB.1.9.2, XBB.2, EG.5.1, BA.4, BA.4.6, BA.5, XBB.2.3, XBB.2.3.2, XBB.1.16.1, XBB.1, FL.3 (XBB.1.9.1.3), XBB.1.5.7, FL.3.1, FL.4, Fl.3.3 ,FY.1.2, BE.3, BQ.l, BQ.1.1 and XBB.1.16) was The results showed that the intensity of the T line at different protein concentrations was consistent with that of the wild strain when tested with the COVID-19 Antigen Rapid Test Cassette.
From the above, it is clear that the COVID-19 Antigen Rapid Test Cassette is effective against virus mutations (B.l.617.2(Delta), BA.1, BA.2, BA2.10, BA.2.12, BA.2.12.1, BA.4, BA.4.6, BA.S, BA.2.75, XE, BE.3, BF.7, BQ.l, BQ.1.1, BJ.l, XBB, BA.2.75.2, BN.l (BA.2.75.5.1), BA.5.2.35, XBB.l.5,CH.1 .1,CHl.l.l, CHl.1.2, XBF(BA.5 x CJ.l), BQ.1.1.20, XBB.1.9.1, XBB.1.9.2, XBB.1.16, EG.l, XBB.2.3, XBB.2.3.2, XBB.1.16.1, XBB.l, FL.3 (XBB.1.9.1.3), XBB.1 .5.7, FL.3.1, FL.4, FL.3.3, FY.1.2, EG.5.l(XBB.1.9.), the risk of the new variant not being detected by the COVID-19 Antigen Rapid Test Cassette is low.
Our company will continue to closely monitor the situation of virus mutations at home and abroad, and timely evaluate the detection ability and product performance of our products on mutated strains to ensure that the accuracy and sensitivity of the COVID-19 Antigen Rapid Test Cassette are not affected. If necessary, the product will be quickly updated to improve the accuracy of the COVID-19 Antigen Rapid Test Cassette on SARS-CoV-2 variants to ensure.
We thank you for your support and trust in our COVID-19 rapid antigen test cassette products.
Safecare Statement on the detection of mutant viruses
Date : November 29th, 2021
Product: SAFECARE Corona Test, 25 Nasal Profi
To all who it may concern,
We, Safecare Biothech (Hangzhou) Co., Ltd. located at Fl.2 Blog.2, No.18 Haishu Road, Hangzhou 311121 China, as a manufacturer of COVID-19 Antigen Rapid Test Kits (Swab) and COVID-19 Antigen Rapid Test Kits (Saliva), hereby declare that our COVID-19 rapid antigen test kits (swabs) and COVID-19 rapid antigen test kits (saliva) for the detection of SARS-CoV-2 antigen even in the event of the newly discovered variantOmicron, which found in South Africa remain effective.
Since the recognition site of the raw materials used in our antigen test is the nucleocapsid protein (nucleoprotein or protein N), which is different from the mutation sites, we would like to explain; that our products theoretically in able to detect this new variant in South Africa.
Finally, We Safecare will strictly implement our quality management system and strive to provide the best products to customers. We will We will also officially inform you if there is any new information about our COVID-19 antigen rapid test kit (swab) and COVID-19 antigen rapid test kit (saliva).
Flowflex Statement for the detection of mutant viruses
Date: 05.01.2023
Product: FLOWFLEX Corona Test, 25 nasal Professional
To all concerned,
Thank you for your interest in the Flowflex SARS-CoV-2 Rapid antigen test.
We, ACON Biotech (Hangzhou) Co., Ltd. as the manufacturer of the Flowflex SARS-CoV-2 rapid antigen test, hereby declare that ACON is observing the emergence of a new SARS-CoV strain , hereby declare that ACON is monitoring the emergence of a new strain of SARS-CoV-2 spreading in the U:K. South Africa, Brazil, Japan, India, USA, Philippines, Peru, Colombia and other countries.
SARS-CoV-2 from Alpha {Bl.1.7), Beta (B.1.35I), Gamma (P.l), Delta (B.1.6I7.2), Epsilon (B.1.427/B.1.429), Zeta (P.2), Eta (B.1.525), Theta (P.3), Iota (B .1.526), Kappa (B.1.6I7.1), Lambda (C.37), Mu (B.l.62I), Delta plus (A Y.4.2), Omicron (B.1.1.529, BA.l, BA. LI, BA.2, BA.2.I2.1, BA.3, XE, BA.4, BA.4.6, BA.5, BA.2.75, BA.2.75.3, BF.l, BF.7, BA.5.1.7, BA.4.6, XBB, XBB.1.5, BE.3, BQ.LI, BS.1, XBC), Bengal (B. I .618) have several mutations in the spike protein and some mutations in the nucleocapsid protein.
When tested with different nucleocapsid protein recombination antigens (alpha, beta, gamma), no obvious difference is seen. Antigens (alpha, beta , Gamma, Delta, Epsilon, Zeta, Eta, Theta, Iota, Kappa, Lambda, Mu, Delta plus, Omicron and Bengals) based on these different variants of SARS-CoV-2, so we do not assume that these variants will have no impact on test performance.
Deepblue statement on the detection of mutant viruses
Date: October 14th, 2022
Product: DEEPBLUE Corona Test 1 saliva layperson
We, Anhui Deepblue Medical Technology Co. located at 4th
Floor, D-1# Zone, Pearl Industrial Park, 106 Innovation Avenue, High-Tech Development Zone, 230088 Hefei, Anhui,China, are here to declare:
As the manufacturer of the product:
- COVID-19 (SARS-CoV -2) Antigen test kit (colloidal gold)
- COVID-19 (SARS-CoV-2) Antigen test kit (colloidal gold) - saliva
- COVID-19 (SARS-CoV -2} Midstream Antigen Test - Saliva
Hygisun statement on the detection of mutant viruses
Date: 29.11.2021
Product: HYGISUN Corona Test, 1 saliva Layman
To whom it may be addressed.
29 November 2021
We, Anbio (Xiamen) Biotechnology Co., Ltd, as the manufacturer of COVID-19 Rapid Antigen Test (Colloidal Gold), hereby declare that the test is effective against mutated strains, including ly, but not limited to the following variants:
SARS-CoV-2 from
- Alpha (Bl.1.7),
- Beta ( B.1.351),
- Gamma (P.l),
- Delta (B.1.617.2),
- Epsilon (B.1.427/B.1.429) ,
- Zeta (P.2),
- Eta (B.1.525),
- Theta (P.3),
- Iota (B.1.526),
- Kappa (B.1.617 .1),
- Lambda (C.37),
- Mu(B.l.621),
- Delta plus (AY.4.2),
- Omicron (B.1.l.529)
No obvious difference is observed when testing with different nucleocapsid proteins Recombination antigens (Alpha, Beta, Gamma, Delta, Epsilon, Zeta, Eta, Theta, Iota, Kappa, Lambda, Mu and Delta plus) based on these different variants of SARS-Co V-2.
At Omicron, the theoretical analysis of mutations in the nucleocapsid protein should support the detection of the COVID-19 antigen rapid test ( colloidal gold). We therefore do not expect that these variants will have no impact on test performance.
Singclean Statement for the detection of mutant viruses
Date: November 30th, 2021
Product: SINGCLEAN Corona Test 5er, Nasal - Layman
To all concerned:
We, Hangzhou Singclean Medteal Products Co., Ltd. as manufacturer of the Singclean COVIO-19 Test Kit (Colloidal Gold Method), hereby declare the following:
Singclean COVI0-19 Test Kit (Colloidal Gold Method) is a solution phase immunochromatographic test for rapid , qualitative detection of antigen of COVID-19 (SARS-CoV-21 in human nasal cavity.
Explain this: Singclean COVID-19 Test Kit (Colloidal Gold Method) is for the detection of SARS-CoV-2 nucleocapsid protein {NP) was determined.
The epitope of the capture antibody and the detection antibody was not in the area of the mutation. Thus, all mentioned spike protein mutations of SARS-CoV-2 and other protein mutations of SARS-CoV-2 can be detected, such as: B.
- (Alpha | B.1.1.7(U.K.));
- (Beta | B.2.351 (South Africa));
- (Gamma |. P.1 (Brazil));
- (Delta | B.1.617.Z(India);
- (Lambda | C.37 (Peru));
- (Zeta | P.Z (Brazil));
- (Kappa | B.1.617,l(India));
- (Eta | B.1.525 (Nigeria));
- (Lota | B.1.526 (U.S.A.));
- (Epsilon | U.,l.427 /8,l.42"9 (U.S.A.)), and
- (Omicron | IU.l.529 (South Africa)).
With the Singclean COVID-19 test kit (colloidal gold method) can detect the above COVID-19 variants.